What is Glycosylation of A Protein
What is Protein Glycosylation
Protein glycosylation denotes the enzymatic coupling of carbohydrate moieties, notably sugars, to predetermined amino acid residues within proteins, thereby yielding glycoproteins. This intricate biochemical phenomenon unfolds within the endoplasmic reticulum and Golgi apparatus, orchestrated by a heterogeneous ensemble of glycosyltransferases. Notably, glycosylation manifests at two primary loci: N-linked glycosylation, wherein glycans are affixed to asparagine residues, and O-linked glycosylation, wherein glycans are appended to serine or threonine residues.
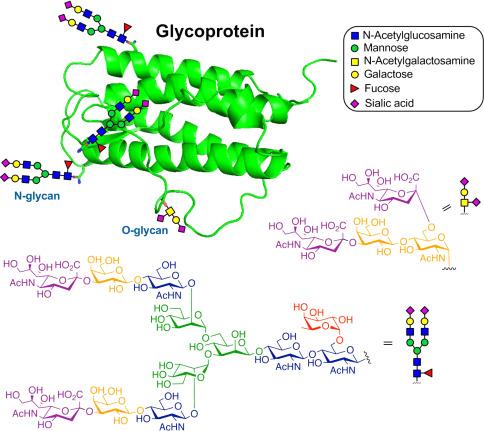
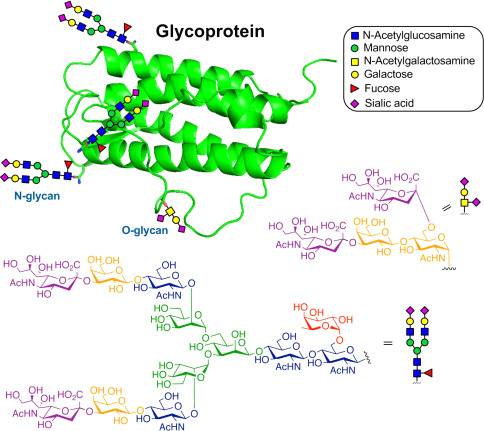 The structure of a typical glycoprotein (Yaohao Li,. et al, Methods in Enzymology, 2019)
The structure of a typical glycoprotein (Yaohao Li,. et al, Methods in Enzymology, 2019)
Understanding the Intricacies of Glycosylation Mechanism
The realm of glycosylation research encompasses the intricate enzymatic operations, substrate discernment, and the governance of quality assurance mechanisms, instrumental in the attachment of glycans to designated molecules. Employing their distinct roles, glycosyltransferases and glycosidases preside over this bioactive process, yielding a broad range of protein structures and functions within cellular entities.
Central to this mechanism is the goal-oriented recognition orchestrated by glycosyltransferases, who execute the anchoring of glycans with unparalleled specificity. Glycosylation is orchestrated at clearly defined amino acid residues, most notably asparagine and serine/threonine residues, which further amplifies the level of complexity within this bioengineered process.
The authoritative pillar in this sequence of events revolves around the proficient inclinations of chaperones and stern quality control protocols, thereby guaranteeing streamlined biosynthesis of glycans. A further testament to the significance of glycosylation is found in its interconnected relations with other modification conduits, subtly delineating its importance in maintaining physiological health and its implications in pathogenesis.
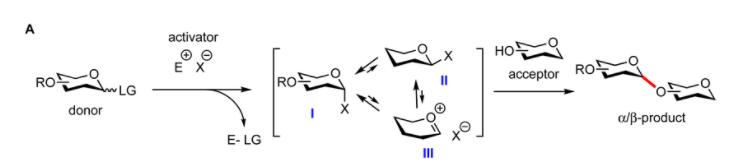 General glycosylation reaction mechanism. (Jacob M. A. van Hengst et al, Chemical Science 2023)
General glycosylation reaction mechanism. (Jacob M. A. van Hengst et al, Chemical Science 2023)
Enzymatic Glycan Attachment
The intricate process of glycosylation, a fundamental post-translational modification, is scrupulously regulated by a diverse array of enzymes, predominantly glycosyltransferases and glycosidases. These respective enzymatic catalysts govern the sequential incorporation and pruning of saccharide residues on specific target molecules, thereby crafting the multifaceted matrix of glycan structures.
A critical step in this dynamic process is catalysed by glycosyltransferases. Their capacity to translocate saccharide moieties from nucleotide sugars to designated acceptors results in the generation of glycosidic bonds between proteins and sugars. This facilitates the consequential and selective attachment of glycans to their respective targets, thereby laying the foundation of glycosylation's complexity.
In a complementary role, glycosidases regulate the streamlining of glycan structures, invoking the hydrolytic cleavage of glycosidic linkages to enable methodical removal of specified saccharide residues. This precise modulation of glycan topology through the concerted interplay of glycosyltransferases and glycosidases underscores the complex yet finely-tuned process of glycan biogenesis and remodeling. This intricate orchestration is integral to expanding the structural heterogeneity and functional spectrum of glycoproteins, reinforcing its significance in both physiological functioning and disease pathogenesis.
Substrate Recognition and Specificity
The glycosylation mechanism hinges significantly on the meticulous recognition and discrimination of substrate molecules by glycosyltransferases. These enzymes assume a pivotal role in facilitating the transfer of sugar moieties onto precise amino acid residues or carbohydrate motifs situated on target proteins. The process of substrate recognition is finely calibrated, showcasing the remarkable specificity of glycosyltransferases towards their substrates.
Mechanisms of Substrate Recognition:
The substrate specificity demonstrated by glycosyltransferases often hinges upon conserved amino acid motifs or binding domains localized within their catalytic regions. These structural elements engage in intricate interactions with distinct features inherent to the substrate protein or carbohydrate moiety, thus ensuring precise glycan attachment. For instance, in N-linked glycosylation, the enzyme oligosaccharyltransferase recognizes the consensus sequence Asn-X-Ser/Thr (where X represents any amino acid except proline) on nascent polypeptide chains for glycan attachment. Similarly, enzymes participating in O-linked glycosylation discern and attach sugars to specific serine or threonine residues within target proteins.
Factors Influencing Substrate Specificity:
Numerous factors underlie the substrate specificity exhibited by glycosyltransferases. Foremost among these is the availability of nucleotide sugar donors, exemplified by UDP-glucose or GDP-mannose, which govern the spectrum of glycan structures amenable to synthesis. The presence or absence of specific sugar donors intricately modulates the range of glycans incorporable into protein substrates. Furthermore, the subcellular localization of glycosyltransferases, notably within organelles like the endoplasmic reticulum or the Golgi apparatus, profoundly influences the precision and efficacy of glycan biosynthesis. Notably, enzymes engaged in initial glycan modifications are primarily sequestered within the endoplasmic reticulum, whereas those participating in intricate glycan elaboration predominantly reside within the Golgi apparatus.
Examples of Substrate Recognition:
An illustrative instance of substrate recognition manifests in the glycosylation of cell surface receptors implicated in cell-cell adhesion. Glycosyltransferases discern specific protein motifs or extracellular domains of these receptors, subsequently modifying them with intricate glycans pivotal for mediating cell-cell interactions. Furthermore, aberrant substrate recognition by glycosyltransferases has been implicated in a spectrum of diseases, encompassing congenital disorders of glycosylation and cancer. For instance, mutations occurring in the substrate-binding domains of glycosyltransferases can induce misglycosylation of proteins, thereby perturbing their cellular functions and contributing to the pathogenesis of diseases.
Post-translational Modification Sites
Glycosylation is a process capable of targeting specific amino acid residues within protein sequences, such as asparagine (N-linked glycosylation) and serine/threonine residues (O-linked glycosylation). The broad spectrum of glycosylation sites, coupled with the structural complexity inherent in glycan structures, significantly enriches the functional diversity observed in glycoproteins.
Functional Implications:
The identification of an array of glycosylation sites along with the inherent structural intricacy of glycans underpins the functional heterogeneity found in glycoproteins. N-linked glycosylation frequently correlates with principal processes such as protein folding, the maintenance of protein stability, and trafficking. O-linked glycosylation, on the other hand, has been associated with pivotal cell activities such as signaling, adhesion, and immune recognition. Notably, anomalous glycosylation at particular sites has been implicated in numerous pathological conditions, underscoring the necessity of elucidating how regulation operates, as well as the repercussions of glycosylation site specificity, in the broader context of human health and disease.
Example of Glycosylation Site Functionality:
A salient illustration regarding the functionality of glycosylation sites can be observed in the N-linked glycosylation process in immunoglobulins. The unvarying Asn-X-Ser/Thr motif, seamlessly integrated within the structure of Immunoglobulin G (IgG), functions as an integral site accommodating glycan attachment.
Through this site-specific glycosylation, the IgG effector functions, inclusive of antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), undergo modulation. This is achieved as the intricate process influences the binding propensity of IgG to Fcγ receptors, as well as complement proteins.
Beyond its biological implications, this glycosylation process also presents substantial potential in relation to therapeutic antibody engineering. This encompasses the elimination or modification of specific glycans at designated sites, which in effect optimizes the potency of the antibody, and subsequently amplifies therapeutic outcomes.
Role of Chaperones and Quality Control Machinery
Indeed, glycosylation, beyond mere enzymatic catalysis, is orchestrated by a sophisticated ensemble of molecular chaperones and quality control apparatus, meticulously safeguarding the accuracy and efficacy of glycan biosynthesis. Molecular chaperones actively participate in orchestrating the precise folding and intracellular trafficking of glycosyltransferases and their substrates, thereby facilitating the intricate process of glycan biosynthesis within the endoplasmic reticulum and Golgi apparatus. Concurrently, quality control mechanisms, exemplified by the unfolded protein response and ER-associated degradation pathways, vigilantly surveil the folding dynamics of glycoproteins and the processing of glycans, preempting the accumulation of misfolded or anomalously glycosylated proteins.
Molecular Chaperones in Glycosylation:
Molecular chaperones play a pivotal role in orchestrating the transit of glycosyltransferases and their substrates through the intricate pathways of protein folding and intracellular trafficking. These specialized proteins establish an environment conducive to the accurate folding of proteins, shielding emerging polypeptides from the perils of misfolding or aggregation. Particularly within the endoplasmic reticulum (ER) and Golgi apparatus, molecular chaperones assume a critical function in directing glycosyltransferases to their designated subcellular locales, thereby facilitating the intricate process of glycan biosynthesis. Through their stewardship of the correct folding of glycosylation enzymes and their substrates, molecular chaperones contribute indispensably to the efficient synthesis of glycoproteins adorned with precisely tailored glycan moieties.
Quality Control Mechanisms:
Beyond the realm of chaperone-mediated folding, quality control mechanisms vigilantly uphold the fidelity of glycoprotein biosynthesis. The unfolded protein response (UPR) and ER-associated degradation (ERAD) pathways represent two principal surveillance systems entrusted with monitoring protein folding and glycan modification processes within the endoplasmic reticulum (ER). In the face of ER stress or disturbances in protein folding, the UPR coordinates a comprehensive transcriptional and translational response aimed at reinstating ER homeostasis and bolstering cellular viability. Concurrently, the ERAD pathway functions as a fail-safe mechanism to eradicate misfolded or irregularly glycosylated proteins from the ER lumen, averting their accumulation and potential cytotoxicity. Through meticulous surveillance and targeted protein degradation, the quality control apparatus ensures the accuracy of glycoprotein assembly and preserves cellular proteostasis.
Evidence from Research:
Scientific investigations have furnished compelling evidence highlighting the pivotal contributions of chaperones and quality control mechanisms to the process of glycosylation. For instance, scholarly inquiries have elucidated the supportive roles of chaperone proteins such as calnexin and calreticulin in guiding the folding and maturation of glycosyltransferases, thereby expediting glycan biosynthesis. Moreover, experimental manipulations involving genetic alterations or pharmacological interventions targeting quality control pathways have consistently yielded outcomes indicative of compromised glycoprotein folding and glycan processing, culminating in the accumulation of misfolded glycoproteins and the onset of pathologies associated with endoplasmic reticulum (ER) stress.
Implications for Disease:
The intricate interplay among chaperones, quality control machinery, and glycosylation pathways carries significant ramifications for human health and disease. Disruption of chaperone function or dysfunction in quality control mechanisms has been linked to a spectrum of protein-folding disorders, encompassing congenital disorders of glycosylation (CDGs) and neurodegenerative ailments such as Alzheimer's and Parkinson's diseases. Comprehending the molecular intricacies governing chaperone-mediated glycosylation quality control offers invaluable insights into disease etiology and unveils potential therapeutic targets for intervention.
Dynamic Regulation and Crosstalk with Other Pathways
The dynamic regulation of protein glycosylation, along with its intricate interconnections with various cellular pathways, underscores its pivotal role in cellular physiology and the pathogenesis of diseases. Delving into the cross-talk between glycosylation and signaling pathways yields valuable insights into the underlying mechanisms of diseases and unveils promising therapeutic avenues for targeted interventions.
Regulatory Mechanisms:
Numerous regulatory mechanisms orchestrate the dynamic landscape of protein glycosylation. Among them, the activity of glycosyltransferases and glycosidases stands prominent, as they catalyze the addition and removal of glycans, respectively. Subject to post-translational modification, allosteric regulation, and precise subcellular localization, these enzymes endow cells with the capacity to finely adjust glycan structures in response to diverse stimuli. Moreover, the availability of nucleotide sugar donors and the expression profiles of genes related to glycosylation exert pivotal influences on the modulation of glycan biosynthesis pathways.
Crosstalk with Signaling Pathways:
Protein glycosylation, through its intricate interplay with numerous cellular signaling pathways, greatly impacts cellular function and behavior. It has been observed that post-translational modification such as glycosylation, can effectively modulate the cellular function by acting upon the activity and subcellular localization of critical signaling receptors, including receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs). This modulation subsequently governs downstream signaling sequences implicated in the processes of cellular proliferation, differentiation, and survival.
Furthermore, a significant role is played by the interactions, facilitated by glycan molecules, between cells and the extracellular matrix (ECM). These interactions are crucial for cell adherence, migration, as well as maintaining tissue homeostasis. Consequently, the role of glycosylation, integral to both developmental processes and the remodeling of tissue, is underscored. Therefore, a comprehensive understanding of the mechanisms driving glycosylation can lead to novel therapeutic strategies to modulate cellular signaling and function.
Functional Implications:
The dynamic control of protein glycosylation bears substantial functional significance in both physiological health and pathological conditions. Dysregulated glycosylation has been implicated across a spectrum of diseases, encompassing cancer, neurodegenerative disorders, and immune dysfunctions. Altered glycan patterns on cell surface receptors and adhesion molecules can disrupt vital intercellular communication and impair immune surveillance, thereby fostering disease progression and pathogenesis. Conversely, strategic manipulation of glycan structures offers promising avenues for therapeutic intervention aimed at ameliorating disease states.
Example of Crosstalk:
An exemplary demonstration of the intricate interplay between protein glycosylation and signaling pathways manifests in the modulation of insulin signaling via O-GlcNAcylation. O-GlcNAcylation, a form of protein glycosylation entailing the addition of O-linked N-acetylglucosamine (GlcNAc) onto serine and threonine residues, dynamically regulates insulin signaling by modifying crucial signaling proteins, notably including insulin receptor substrate 1 (IRS-1) and Akt. O-GlcNAcylation of IRS-1 impedes its phosphorylation by insulin receptor tyrosine kinases, consequently blunting insulin signaling and contributing to the development of insulin resistance, a hallmark feature of type 2 diabetes mellitus.
Deciphering Protein Glycosylation Sites
Two major types of protein glycosylation prevail: N-linked glycosylation and O-linked glycosylation.
N-Linked Glycosylation Sites
N-linked glycosylation, a prevalent post-translational modification, predominantly targets asparagine (Asn) residues within specific amino acid motifs. This modification is executed at the consensus sequence Asn-X-Ser/Thr, where X represents any amino acid except proline. Extensive analyses of protein sequences alongside experimental data have facilitated the identification of numerous N-linked glycosylation sites. Deciphering the sequence determinants of N-linked glycosylation holds paramount importance in elucidating its functional ramifications, encompassing protein folding, stability, and cellular function.
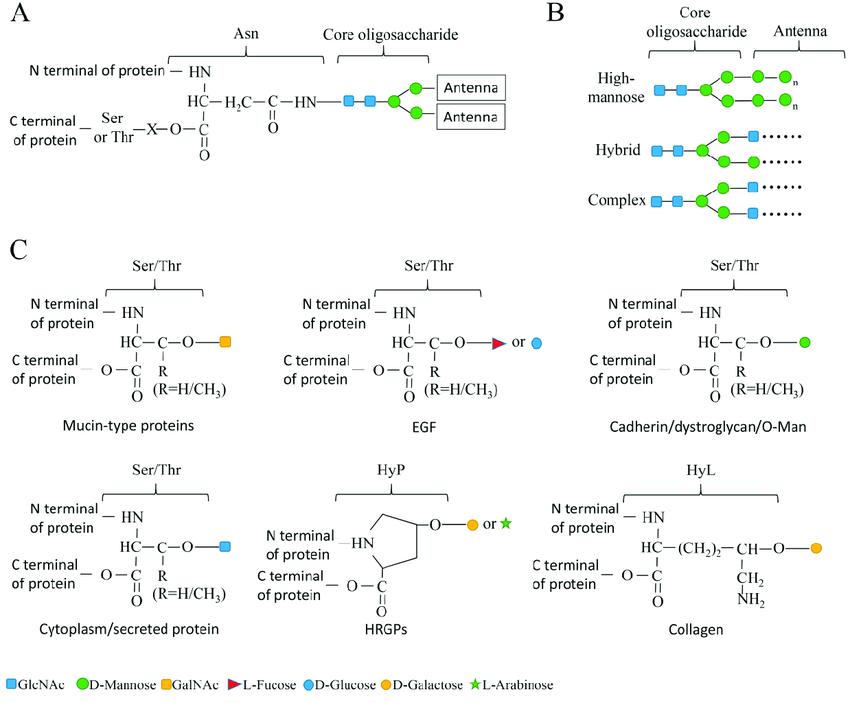 Diagram showing the structure of N-linked and O-linked glycosylation types (Borong Lin et al,. Cells 2020)
Diagram showing the structure of N-linked and O-linked glycosylation types (Borong Lin et al,. Cells 2020)
O-Linked Glycosylation Sites
O-linked glycosylation sharply contrasts its N-linked counterpart, authentically targeting the serine (Ser) and threonine (Thr) amino acid residues embedded in protein sequences. Oftentimes, the sheer genetic complexity, as seen in the variability of sequence motifs within O-linked glycosylation, presents researchers with considerable impediments when tasked with its identification and characterization. Nevertheless, recent advancements, particularly in bioinformatic and mass spectrometry-based analysis, have provided scholars with indispensable tools to map O-linked glycosylation sites with precision across an array of proteomes. Dissecting the complexity inherent in O-linked glycosylation continues to yield invaluable insights into the intricate network of regulatory mechanisms as well as the functional repercussions within cellular systems.
Site-Specific Analysis and Functional Implications
Approaches employing site-specific glycoproteomics are invaluable in conducting a thorough characterization of glycosylation sites, and investigating their associations with biological functions. The integration of data obtained from mass spectrometry-based glycoproteomics with predictions of protein structure, coupled with functional annotations, substantively enhances our comprehensive understanding of the roles played by glycosylation sites. Through identification of glycosylation 'hotspots' and decisive residues, it becomes feasible for scientists to elucidate their involvement in the orchestration of protein-to-protein interactions, signal transduction pathways, and the etiology of various diseases. This essential research thus affords a profound understanding of pivotal biological mechanisms and provides a clear basis for the design of therapeutic strategies.
Dynamic Regulation and Contextual Specificity
The capacity for dynamic regulation of glycosylation sites is evident through their responsiveness to cellular stimuli, cues during biological development, and pathological conditions, underscoring the context-dependent nature of protein glycosylation. A deeper examination of the shifting topography of glycosylation sites in circumstances of wellness and illness allows for the sophisticated exploration of the interactions between glycan biosynthesis, protein functionality, and the overall physiological performance of cells. By elucidating the intricate control mechanisms behind the occupancy of glycosylation sites and the morphology of glycans, we amplify our comprehension of pathology and guide the progression towards the creation of tailored therapeutic strategies.
Future Directions and Technological Innovations
As the field of glycoproteomics steadily progresses, researchers are actively pursuing innovative technologies to propel advancements in glycosylation site mapping and functional elucidation. The integration of state-of-the-art proteomics platforms, sophisticated bioinformatics algorithms, and advanced structural biology techniques empowers investigators to untangle the intricacies inherent in protein glycosylation. Through the meticulous deciphering of the elaborate network of glycosylation sites and their associated functional implications, researchers aspire to formulate precision medicine strategies capable of addressing an extensive spectrum of health and disease conditions.
Protein Glycosylation Function: Deciphering its Functional Significance
Protein glycosylation emerges as a pivotal regulator in governing diverse facets of cellular physiology, spanning protein folding, stability, and intercellular communication. Within the investigative realm of Creative Proteomics, we undertake a comprehensive exploration into the functional implications of protein glycosylation, elucidating its manifold roles in both physiological homeostasis and pathological states.
Facilitating Protein Folding and Stability
Protein glycosylation stands as a pivotal factor governing the fidelity of protein folding and stability, thereby orchestrating the generation of biologically active proteins. Conjugated glycan entities, affixed to nascent polypeptide chains, assume the role of molecular chaperones, directing the intricate folding trajectory and forestalling misfolding or aggregation events. This surveillance mechanism finds poignant illustration in investigations probing the involvement of glycosylation in protein folding disorders, such as cystic fibrosis. Notably, seminal research documented in the esteemed journal Biochemistry (Stanton et al., 1995) elucidated the indispensability of N-glycosylation in orchestrating the proper folding and cellular trafficking of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Furthermore, perturbations in CFTR glycosylation resultant from mutations in the cystic fibrosis gene engender protein misfolding and subsequent degradation, underscoring the pivotal role of glycosylation in safeguarding protein quality control mechanisms.
Modulating Protein-Protein Interactions
Protein glycosylation emerges as a central orchestrator in orchestrating protein-protein interactions, thereby intricately regulating fundamental cellular processes such as cell-cell adhesion and signaling. The presence of glycan moieties on cell surface receptors and adhesion molecules assumes pivotal significance as recognition sites for ligands, thereby facilitating the intricate choreography of cell-cell recognition and communication. Landmark investigations, as documented in the esteemed journal Cell (Varki, 2017), underscore the critical role of glycosylation in fine-tuning the interactions between cell surface receptors and their extracellular ligands. Notably, the glycosylation status of integrins, a prominent family of cell adhesion molecules, emerges as a determinant influencing their binding affinity to extracellular matrix proteins, thereby exerting precise control over cell adhesion dynamics and migratory behavior. Moreover, the capacity of glycosylation to modulate both the avidity and specificity of protein-protein interactions finds compelling validation in scholarly discourse, as exemplified by seminal research delineated in Nature Communications (Pinho and Reis, 2015) elucidating the nuanced involvement of glycosylation in shaping immune cell signaling cascades.
Serving as Immune Modulators
Protein glycosylation emerges as a critical orchestrator in the modulation of immune responses, intricately shaping processes of immune recognition and subsequent responses. The attachment of glycans to cell surface antigens and immunoglobulins assumes pivotal significance in the landscape of immune recognition and response dynamics. Seminal investigations, as chronicled in the scholarly journal Immunity (Crocker et al., 2007), elucidate the profound impact of glycosylation on the functionality of immune cell surface receptors, exemplified by Siglecs, thus intricately modulating immune cell signaling and function. Furthermore, the structural alterations in glycan moieties wield substantial influence over antigen presentation and antibody-mediated immune responses, as unveiled by scholarly discourse in the Journal of Experimental Medicine (Kaneko et al., 2006), particularly in the context of autoimmune pathologies.
Implications in Disease Pathogenesis
The dysregulation of protein glycosylation emerges as a significant contributor to diverse disease pathologies, encompassing cancer, neurodegenerative disorders, and metabolic ailments. Perturbed glycosylation profiles observed on cell surface receptors, enzymes, and extracellular matrix proteins precipitate disruptions in normative cellular functions, thereby fostering disease progression and pathogenicity. Scholarly discourse, as documented in Nature Reviews Cancer (Pinho and Reis, 2015), accentuates the pivotal involvement of altered glycosylation patterns in the intricate processes of cancer metastasis and immune evasion. Furthermore, investigations delineated in the Journal of Alzheimer's Disease (Arnold et al., 2018) elucidate the nuanced role of glycosylation in the pathogenesis of Alzheimer's disease, thus unveiling potential avenues for therapeutic intervention in this debilitating neurological condition.
Exploring Therapeutic Opportunities
Considering the pivotal significance of protein glycosylation in both physiological health and disease pathology, the strategic targeting of glycan-mediated processes emerges as a promising frontier for therapeutic intervention. A surge of innovative glycan-based therapeutic modalities is actively under investigation across diverse disease landscapes, encompassing malignancies such as cancer and autoimmune afflictions. Scholarly discourse, as illuminated in Nature Reviews Drug Discovery (Dennis et al., 2019), underscores the transformative potential inherent in glycoengineering strategies for the development of precisely targeted therapeutics.
The Difference Between Glycosylation and Glycation
Distinguishing between glycosylation and glycation elucidates fundamental disparities in their biochemical processes and biological ramifications. Glycosylation represents a precisely orchestrated post-translational modification orchestrated by enzymes, wherein a specific carbohydrate moiety is affixed to a predetermined region of the protein. Despite its inherent heterogeneity, protein glycosylation operates as a regulated mechanism, endowing living cells with distinct functional properties.
In contrast, glycation, often erroneously conflated with glycosylation, manifests as a stochastic process occurring within the bloodstream. This non-enzymatic reaction involves the covalent attachment of the reducing ends of free sugars—such as glucose, fructose, or galactose—to proteins, resulting in the formation of glycated products. Glycation detrimentally affects protein function and stability, thereby bearing relevance to numerous disease processes. For instance, in diabetic individuals, the quantification of glycated hemoglobin serves as a marker reflecting elevated glucose levels over prolonged durations, underscoring the clinical significance of glycation in disease pathology.
References
- Arnold, J. N., Saldova, R., Galligan, M. C., Murphy, T. B., Mimura-Kimura, Y., Telford, J. E., ... & Rudd, P. M. (2008). Novel glycan biomarkers for the detection of lung cancer. Journal of proteome research, 7(4), 1167-1175.
- Crocker, P. R., Paulson, J. C., & Varki, A. (2007). Siglecs and their roles in the immune system. Nature Reviews Immunology, 7(4), 255-266.
- Dennis, J. W., Granovsky, M., & Warren, C. E. (1999). Glycoprotein glycosylation and cancer progression. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1473(1), 21-34.
- Kaneko, Y., Nimmerjahn, F., & Ravetch, J. V. (2006). Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science, 313(5787), 670-673.
- Pinho, S. S., & Reis, C. A. (2015). Glycosylation in cancer: mechanisms and clinical implications. Nature Reviews Cancer, 15(9), 540-555.
- Stanton, B. A., Guggino, W. B., Hug, M. J., & Thomas, P. J. (1995). Mechanisms of cftr activation by glycerol derivatives in planar lipid bilayers. Biochemistry, 34(14), 4256-4265.
- Varki, A. (2017). Biological roles of glycans. Glycobiology, 27(1), 3-49.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


